
Today (NST)
Friday, Apr 25, 2025
Smart Search
Please post in your Technical Queries, Comments & Suggestions to Contact us......
Advertisement

For Advertisement
|
|
| Subscribe to BREINS Sci-Tech |
| Visit this group |

Concrete Technology
Concrete, the most widely used construction material can also be called as universal material of construction with its growing demand worldwide due to its versatility and easy availability of its constituent materials. Concrete being a man made material of construction primarily constitutes of following ingredients: Gravel, Sand, Cement & water. Agggregates: Gravel & Sand almost constitute more than 70% by volume of concrete.
Concrete technology refers to all those processes involved in production of “quality concrete” that performs satisfactorily in the fulfillment of strong & durable structures. Concrete being a mixture of naturally available materials and with so many variable factors in its production; producing a concrete of desired strength & durability is indeed a challenging job that needs a stringent quality control measures.

Fig-1 (Failure of RC Column due to poor concrete quality)
Quality control begins right from the material selection phase; such as selection of cement, gravel, sand and water itself. Selection of each of the above ingredients demands various kinds of tests for its approval.
Cement
Portland cement, commonly known as “cement”, is an inorganic material
obtained by mixing together materials such as limestone, sand, clays
and iron ores; burning them at a clinkering temperature and grinding
the resulting clinker. It develops strength by chemical reaction with
water by formation of hydrates. Hydrate products form the binding component
that binds together building blocks of concrete: gravels. So cement
quality largely determines the strength and durability of overall concrete
itself. In such various properties of cement need to be checked while
approving it for a project.
Major Chemical Compounds
in Cement
Main chemical compounds of Portland cement with their chemical formula
& their percentages by weight are as follows.
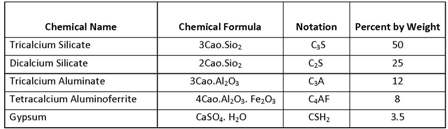
Of the all, the silicates C3S and C2S are the most important compounds, which are responsible for the strength of hydrated cement paste. The presence of C3A in cement is undesirable. C4AF is also present in cement in small quantities and compared with other three compounds, it does not affect the behavior significantly. Gypsum prevents the flash set of cement.
C3S - Hydrates and hardens rapidly and is responsible for initial set and early strength. Portland cements with higher percentages of C3S will exhibit higher early strength.
C2S - Hydrates and hardens slowly and is largely responsible for increase in strength beyond 7 days.
C3A - Hydrates and hardens the quickest. Liberates a large amount of heat almost immediately and contributes somewhat to early strength.
C4AF - Hydrates rapidly but contributes very little to strength. Its use allows lower kiln temperature in Portland cement manufacturing. Most Portland cement color effects are due to C4AF.
CSH2 - Without Gypsum, C3A hydration would cause Portland cement to set almost immediately after addition of water.
Hydration of Cement
During hydration, cement reacts with water liberating heat and forming
products like Calcium Silicate Hydrates and Calcium Hydroxide. Following
reactions occur:
a) Hydration of Tricalcium Silicate
2 (3CaO.SiO2) + 6H2O → 3CaO.2SiO2.3H2O + 3Ca(OH)2
2C3S + 6H → C3S2H3 + 3Ca(OH)2
2C3S + 6H → C-S-H (Cement Gel) + 3Ca(OH)2
100 + 24 → 75 + 49 (by weight)
b) Hydration of Dicalcium Silicate
2 (2CaO.SiO2) + 4H2O → 3CaO.2SiO2.3H2O + 3Ca(OH)2
2C2S + 4H → C3S2H3 + 3Ca(OH)2
2C2S + 4H → C-S-H (Cement Gel) + 3Ca(OH)2
100 + 21 → 99 + 22 (by weight)